
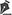
Daniel Perez-Ajami1; Elisa Viscasillas Navarro2
DOI: 10.5935/2595-170X.20250008
ABSTRACT
Burns from chlorine trifluoride are exceptionally uncommon and pose a complex clinical challenge due to combined thermal, chemical, inhalational, and systemic toxicity. We report a middle-aged hypertensive smoker who sustained extensive facial, cervical, and upper-limb burns following an industrial ClF3 leak. He underwent early endotracheal intubation, tailored fluid resuscitation guided by urine output, and calcium gluconate therapy to neutralize fluoride ions. His course was complicated by rhabdomyolysis-associated renal dysfunction, acute lung injury, metabolic disturbances, and wound infection managed with broad-spectrum antibiotics and staged surgical debridement with skin grafting. This case highlights the necessity of prompt airway protection, precise hemodynamic management, aggressive electrolyte correction, renal support, and a coordinated multidisciplinary approach to optimize outcomes in complex chemical burns.
Keywords: Burns, Chemical. Critical Care. Patient Care Team.
RESUMO
Queimaduras por trifluoreto de cloro são extremamente raras e apresentam grande desafio clínico, combinando lesão térmica, química, por inalação e toxicidade sistêmica. Relatamos um paciente hipertenso e tabagista de meia-idade com queimaduras extensas em face, pescoço e membros superiores após vazamento industrial de ClF3. Foram realizadas intubação precoce, ressuscitação volêmica ajustada ao débito urinário e terapia com gluconato de cálcio para neutralização de fluoretos. A evolução incluiu rabdomiólise com disfunção renal, lesão pulmonar aguda, distúrbios metabólicos e infecção das feridas, tratadas com antibioticoterapia de amplo espectro, desbridamentos seriados e enxertia de pele. Este relato reforça a importância de proteção rápida das vias aéreas, manejo hemodinâmico preciso, correção eletrolítica agressiva, suporte renal e abordagem multidisciplinar na otimização de resultados em queimaduras químicas complexas.
Palavras-chave: Queimaduras Químicas. Cuidados Críticos. Equipe de Assistência ao Paciente.
INTRODUCTION
Chemical burns are uncommon but often severe, especially when caused by highly reactive compounds. Chlorine trifluoride (ClF3) is an aggressive halogenating agent used in some industrial processes (e.g. semiconductor manufacturing) that reacts violently with water and organic material. Upon contact with tissues or moisture, ClF3 liberates hydrofluoric acid (HF) and other oxidizing species, causing simultaneous deep thermal and chemical injuries1. Fluoride ions penetrate tissues and bind calcium and magnesium, producing liquefactive necrosis and life-threatening systemic toxicity2. Inhalation of ClF3 vapors can induce airway inflammation, mucosal necrosis and pulmonary edema3. Because of these combined effects, ClF3 burns pose a unique clinical challenge that requires aggressive resuscitation and multidisciplinary care. We report the case of a 59-year-old man who suffered extensive burns after ClF3 exposure, highlighting the clinical course, laboratory findings, and management of fluoride ion toxicity and severe inhalation injury.
CASE REPORT
A 59-year-old man with no personal history other than hypertension and a 20-pack-year smoking history was admitted to the Burn Intensive Care Unit 1 hour after an industrial accident. He had been exposed to a sudden ClF3 gas leak in a chemical research laboratory. On arrival, he was alert but in distress. Physical examination revealed burn injuries covering 21% of total body surface area (TBSA) - principally the face, neck, and bilateral upper extremities (Figure 1). Burns over the face and anterior neck appeared deep partial-thickness (second-degree) with leathery gray eschar, while peripheral areas on the arms showed mixed partial- and full-thickness injury. Carbonaceous sputum was present in the mouth. Initial vital signs indicated shock and respiratory compromise: blood pressure 85/55 mmHg, heart rate 145 bpm, respiratory rate 30/min, and oxygen saturation 82% on 100% non-rebreather mask. He had inspiratory stridor and hoarseness, suggestive of inhalation injury.
Arterial blood gas on 100% oxygen showed hypoxemia (PaO2 70 mmHg) and metabolic acidosis (pH 7.28, HCO3- 16 mmol/L, lactate 5.5 mmol/L). Initial laboratory tests revealed hemoglobin 15.2 g/dL, WBC 18,000/µL, serum creatinine 1.0 mg/dL, BUN 15 mg/dL, potassium 4.8 mmol/L, ionized calcium 1.05 mmol/L (normal 1.12-1.32), and normal liver enzymes. Creatine kinase (CK) was mildly elevated to 800 U/L. He received an immediate tetanus booster, and broad-spectrum antibiotics were drawn for later adjustment.
Within minutes of arrival, the patient was intubated prophylactically via orotracheal tube under rapid sequence induction due to the risk of progressive airway edema. Fiberoptic bronchoscopy confirmed soot and mucosal erythema down to the carina. An arterial line and central venous catheter were placed. Aggressive fluid resuscitation was started using a modified Parkland formula (lactated Ringer's solution, 4 mL/kg per %TBSA in 24 h, half in first 8 h), titrated to maintain urine output ≥0.5 mL/kg/h. Despite 8 L of fluids in the first 12 hours, his blood pressure remained low, and he required norepinephrine infusion (up to 0.2 µg/kg/min) for hemodynamic support.
Given the chemical nature of the burn, calcium gluconate therapy was initiated both topically and intravenously. A 2.5% calcium gluconate gel was applied to affected areas on the hands and arms, and an IV infusion of calcium gluconate (1 g/h) was started. Aggressive decontamination was performed by copious irrigation, and care was taken to protect providers from exposure (full PPE). Enteral access was established early, and high-calorie, high-protein nutritional support was begun on day 1.
By 24 hours post-injury, his condition evolved with worsening metabolic derangements. The patient became oliguric with dark-colored urine. Repeat labs at 48 h showed CK 22,000 U/L, serum myoglobin >1,500 µg/L, and creatinine 3.6 mg/dL, consistent with rhabdomyolysis and acute kidney injury (AKI). Serum ionized calcium had fallen to 0.85 mmol/L (corrected Ca 5.8 mg/dL), and total serum protein (albumin) dropped to 2.1 g/dL. Serum potassium rose to 5.6 mmol/L, and bicarbonate fell to 12 mmol/L (pH 7.18). These changes reflected fluoride-mediated mineral binding, third-spacing from burns, and burn shock. Continuous renal replacement therapy (CRRT) was initiated on ICU day 2 for refractory hyperkalemia, acidosis, and volume management. Calcium and magnesium were replaced aggressively; ECG monitoring showed QT prolongation at 24 hours (QTc 520 ms), which improved with IV calcium.
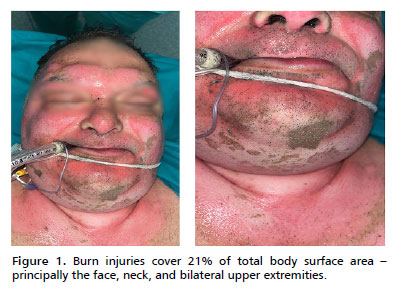
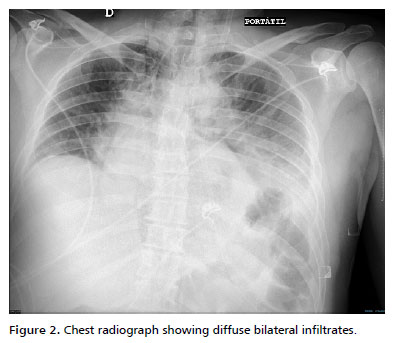
Respiratory status deteriorated by day 2. Chest radiograph showed diffuse bilateral infiltrates. On ventilatory support (volume-control mode, tidal volume 6 mL/kg), his PaO2/FiO2 ratio was 140, consistent with moderate Acute Respiratory Distress Syndrome (ARDS) (Figure 2). A lung-protective strategy with high PEEP (up to 14 cmH2O), low tidal volume, and neuromuscular blockade was applied. He remained sedated (midazolam and fentanyl infusions) and paralyzed (cisatracurium) for 48 h. Inhaled bronchodilators and nebulized 2.5% calcium gluconate were administered to mitigate airway inflammation. Diuretics were avoided during early resuscitation but used later to achieve negative fluid balance once stabilized, thereby promoting resolution of pulmonary edema and contributing to the recovery from ARDS (Figure 3).
Burn wound care included gentle debridement and topical 0.5% silver sulfadiazine twice daily. By day 5, surgical consultation led to escharotomies of circumferential neck burns to relieve constriction, followed by staged excisional debridement. Subsequent wound cultures (day 7) grew Pseudomonas aeruginosa, and piperacillin-tazobactam was given for 10 days. Over the next two weeks, he underwent serial debridements (days 5, 10, 16) and autologous split-thickness skin grafting of the face and arms.
In the ensuing ICU course, the patient's metabolic status gradually normalized. By day 10, urine output improved with recovering renal function (creatinine 1.4 mg/dL), allowing discontinuation of CRRT on day 12. Calcium supplements continued for persistent hypocalcemia until day 14 when serum calcium stabilized at 8.0 mg/dL without IV supplementation. He was weaned off vasopressors by day 8 and extubated on day 14 after fulfilling weaning criteria (PaO2 95 mmHg on 40% O2, FiO2; P/F ratio >250). High-protein enteral nutrition was maintained, correcting hypoproteinemia (albumin 3.0 g/dL by day 15). Intensive physiotherapy and wound care continued, and the patient was transferred to the burn ward on day 25 with most grafts taking well and no new complications. At discharge from ICU, he was ambulatory with supplemental calcium and vitamin D, and outpatient follow-up was arranged.
DISCUSSION
ClF3 burns combine deep chemical and thermal injury with systemic toxicity. Upon hydrolysis, HF releases fluoride ions that bind calcium and magnesium, depleting serum levels and inducing cellular ATPase inhibition, membrane disruption, and liquefactive necrosis2. Systemic absorption causes hypocalcemia, hypomagnesemia, hyperkalemia, metabolic acidosis, and arrhythmias1. In severe HF burns, refractory ventricular fibrillation has been reported when fluoride load is high4. Early ECG monitoring and aggressive calcium repletion are therefore essential2,4. We administered continuous IV and topical calcium gluconate consistent with HF protocols1,2.
Burn shock from capillary leak and third-spacing necessitates precise fluid resuscitation. While guidelines recommend the Parkland formula, invasive hemodynamic monitoring (arterial pulse contour, CVP) may optimize therapy and avoid fluid overload that exacerbates ARDS4. Our use of early vasopressors alongside fluids-maintained perfusion without worsening pulmonary edema.
Inhalation injury poses high mortality risk. Prophylactic intubation at first signs of facial burns or stridor is endorsed by burn consensus guidelines5. Fiberoptic bronchoscopy confirmed injury extent and assisted in timing extubation, which was achieved on day 14 without airway stenosis. Nebulized calcium gluconate has shown benefit in chemical inhalation injuries5.
Rhabdomyolysis-induced AKI is common in major burns with muscle involvement. CK and myoglobin elevations correlate with AKI risk6,7. Early CRRT (within 24 h of diagnosis) facilitates myoglobin clearance, corrects electrolytes, and manages volume, contributing to renal recovery in our patient.
Infection remains a major complication. Early excision and grafting reduce bacterial colonization and improve survival2,8. Our multidisciplinary approach-engaging intensivists, toxicologists, nephrologists, burn surgeons, and rehabilitation therapists-reflects modern burn care models4.
Emerging therapies such as nanofiber dressings, bioengineered skin substitutes, and systemic anti-inflammatory agents show promise in accelerating healing and modulating the inflammatory response9. Although not applied here, these advances may further improve outcomes in ClF3 burns.
FINAL CONSIDERATIONS
Burns from chlorine trifluoride are exceptionally rare and devastating, combining the features of deep chemical burns with multi-organ toxicity. This case illustrates the critical importance of anticipating and treating the systemic effects of fluoride ion, including rapid correction of hypocalcemia and metabolic derangements. Early airway protection, judicious fluid resuscitation with invasive monitoring, and prompt renal support proved life-saving. Our experience reinforces that such patients require management in a specialized burn center with a coordinated multidisciplinary team. Continued accumulation of case data is needed to develop evidence-based protocols for ClF3 injuries. Ultimately, prompt recognition of chemical inhalation, intensive organ support, and novel therapies (e.g. advanced dressings or systemic detoxification agents) may improve outcomes in these rare but challenging injuries10.
REFERENCES
1. Bajraktarova-Valjakova E, Korunoska-Stevkovska V, Georgieva S, Ivanovski K, Bajraktarova-Misevska C, Mijoska A, et al. Hydrofluoric Acid: Burns and Systemic Toxicity, Protective Measures, Immediate and Hospital Medical Treatment. Open Access Maced J Med Sci. 2018;6(11):2257-69. DOI: https://doi.org/10.3889/oamjms.2018.429
2. Walsh K, Hughes I, Dheansa B. Management of chemical burns. Br J Hosp Med (Lond). 2022;83(3):1-12. DOI: https://doi.org/10.12968/hmed.2020.0056
3. Radzikowska-Büchner E, Lopuszynska I, Flieger W, Tobiasz M, Maciejewski R, Flieger J. An Overview of Recent Developments in the Management of Burn Injuries. Int J Mol Sci. 2023;24(22):16357. DOI: https://doi.org/10.3390/ijms242216357
4. Shen Y, Fu L, Yue Z, Shi W, Li C, Zhang C. First aid treatment and airway management for chemical burns combined with inhalation injury: A case report. Respirol Case Rep. 2024;12(12):e70094. DOI: https://doi.org/10.1002/rcr2.70094
5. Concannon E, Damkat Thomas L, Kerr L, Damkat I, Reddi B, Greenwood JE, et al. Review of Indications for Endotracheal Intubation in Burn Patients with Suspected Inhalational Injury. Eur Burn J. 2023;4(2):163-72. DOI: https://doi.org/10.1016/10.3390/ebj4020014
6. Ko A, Song J, Golovko G, El Ayadi A, Ozhathil DK, Wermine K, et al. Higher risk of acute kidney injury and death with rhabdomyolysis in severely burned patients. Surgery. 2022;171(5):1412-6. DOI: https://doi.org/10.1016/j.surg.2021.09.029
7. Stollwerck PL, Namdar T, Stang FH, Lange T, Mailänder P, Siemers F. Rhabdomyolysis and acute renal failure in severely burned patients. Burns. 2011;37(2):240-8. DOI: https://doi.org/10.1016/j.burns.2010.09.009
8. National Academies of Sciences. Burns. In: Health Hazard Assessment of Chemical Substances (NIOSH CIB 66). Washington: U.S. Department of Health and Human Services; 2013.
9. Palao R, Monge I, Ruiz M, Barret JP. Chemical burns: pathophysiology and treatment. Burns. 2010;36(3):295-304. DOI: https://doi.org/10.1016/j.burns.2009.07.009
10. Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, et al. Burn injury: Challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3-17. DOI: https://doi.org/10.1016/j.addr.2017.09.018
Recebido em
4 de Janeiro de 2025.
Aceito em
20 de Maio de 2025.
Place of work: Hospital La Fe, Valencia, Espanha.
Conflict of interest: The authors declare that there is none.